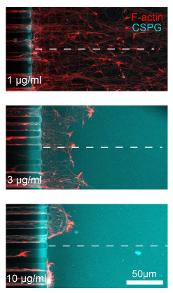
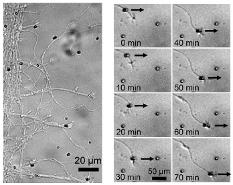
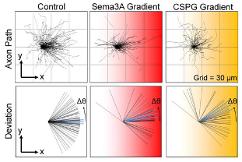
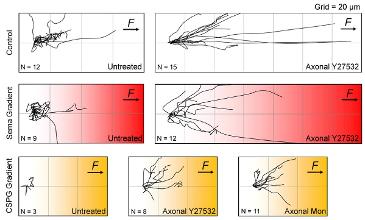
- Axons subjected to Semaphorin 3A (Sema3A) and chondroitin sulfate proteoglycans (CSPG) gradients slow down and deviate from their elongation direction.
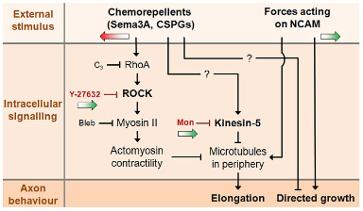
- Inhibition of axonal Rho kinase (ROCK) and calpain blocks the effects of Sema3A. Inhibition of ROCK and kinesin-5 blocks the effects of CSPG.
- Growth cone pulling with less than 12 pN force acting on NCAMs successfully towed axons against Sema3A gradients only when axons were co-treated with ROCK inhibitors.
- Similarly, axons were towed against CSPG when they were co-treated with inhibitors of ROCK and kinesin-5.
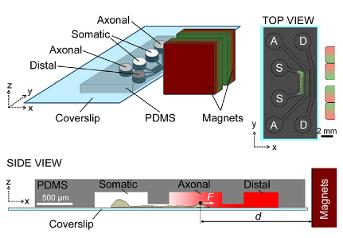
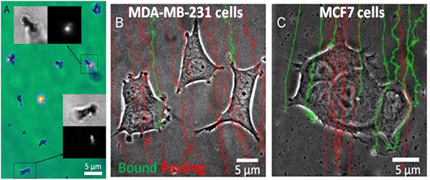
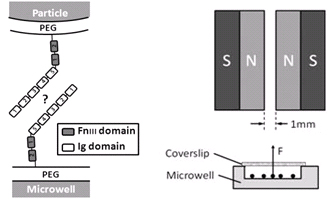
Methods: Fe segments were coated with PEG. Au tips were functionalized with Streptavidin (STR) or with Heregulin (HRG). Cell targeting was tested under flow conditions in a microfluidic channel. Magnetic tweezers forces were applied and ERK phosphorylation was assessed using immunocytochemistry.
- FITC-biotin labeling of STR-conjugated nanorods confirms tip functionalization (A).
- MCF7 cells express ErbB2 receptors 2.3× higher in MCF7 cells.
Accordingly, HRG-rods binding to MCF7 is 2.2× higher than MDA cells (B-C). Also see Movie S1.
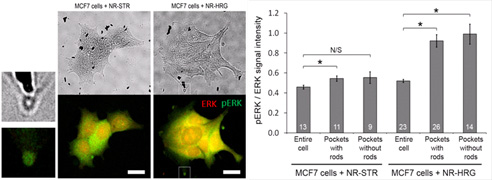
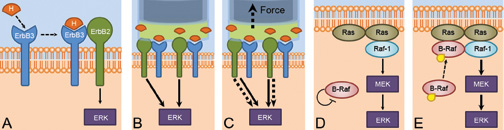
- MCF7 cells targeted with HRG-nanorods exhibit focal ERK phosphorylation. These
‘active zones’ were also targeted preferentially, 6×
more often than the rest of the cell.
- Periodic
mechanical stretch (0.5 Hz) enhances ERK phosphorylation in targeted cells regardless of the force magnitude (4.1 pN -
12.7 pN). Also see Movie S6. At high forces, rods formed membrane tethers.
- Low dose B-Raf inhibition also enhances ERK activation and kills MCF7 cells when combined with periodic mechanical stretch.
- Magnetic hyperthermia and subsequent cell death can be induced with very weak alternating magnetic fields and at low particle densities (not shown).
Major Findings:
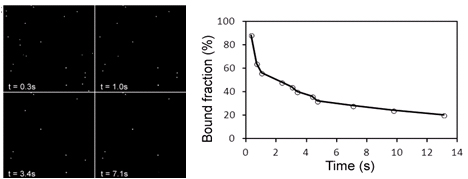
- External force decreases bond lifetime as a function of force magnitude, surface density, and binding period.
- Simulations based on Bell model, koff = koff,0 · exp(Fext/f), favor a bond model with two types of bonds
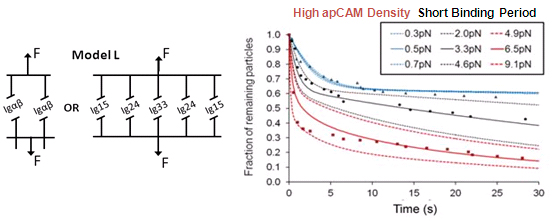
This study was the first to pull on Ig-CAM bonds with constant, physiologically relevant forces. Bond dissociation rate increased according to Bell model. Magnetic tweezers–based force clamp was shown to be suitable method to characterize weak molecular interactions.
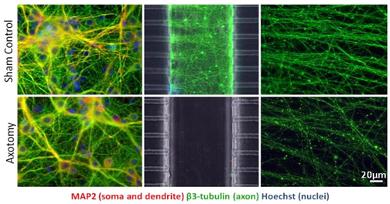
Major Findings:
- Axotomy induces progressive degeneration in distal axons, but no cell death in 24h. Degeneration is asynchronous and progresses anterograde.
- Axotomized axons were treated with post-injury calpain and caspase inhibitors and pre- and post-injury βNAD. Wallerian-like degeneration is delayed with βNAD but not with calpain nor caspase inhibitors.
Significance:
Our model enables simultaneous mass axotomy of CNS axons and permits the treatment of isolated segments along the axons.
Wallerian-like
degeneration can be blocked by pre-treating with βNAD, a molecule
associated with mitochondrial energy metabolism and neurodegenerative
diseases.

Background: Human diffuse axonal injury (DAI) is a result of closed head trauma and exhibits
- increased membrane permeability
- increased [Ca2+] and calpain activity
- cytoskeletal damage and impaired axonal transport
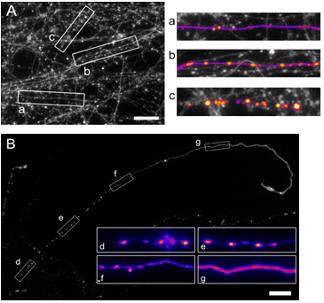
- axonal beading, the “hallmark” morphology of DAI
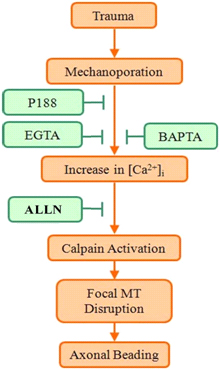
Hypothesis: Injury increases axonal beading via mechanoporation-induced, calpain-mediated disruption of microtubules.
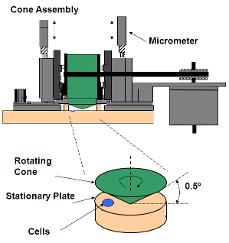
Methods: We applied fluid shear stress injury to cultured central nervous system (CNS) neurons in order to mimic axon degeneration following DAI. To test the hypothesis, we
- treated injured neurons with Poloxamer 188 (P188), a triblock copolymer that reseals damaged membranes
- chelated intra- and extra-cellular Ca2+ using BAPTA and EGTA, respectively
- inhibited calpain activity using its specific inhibitor ALLN
Major Findings:
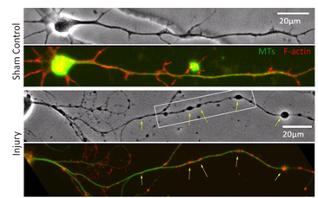
- Injury induces membrane poration and gradual axonal beading. Post-injury P188 application blocks both responses. Focal microtubule disruption underlies bead formation.
- Injury induces increases in [Ca2+]I and calpain which are blocked by Ca2+ chelation, calpain inhibition, and by post-injury P188 treatment.
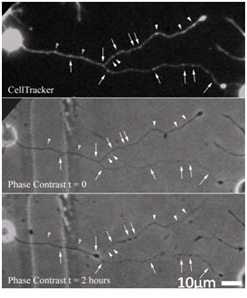
- Axonal mitochondria (“hot spots” in CellTracker image of uninjured axon) predict future bead locations.
Significance:
Our model mimics important features of
DAI including mechanoporation, microtubule disruption, and axonal
beading. We provide evidence that Ca2+-mediated calpain activity is a
crucial step in the DAI neuropathology. The co-localization of the Ca2+
hot spots, mitochondria, and future bead locations suggests that
mitochondria play an important role in the focal cytoskeletal damage
following injury. Finally, we propose P188 as a membrane therapy for
traumatic injury.